Which of the Following Atoms Has the Greatest Nuclear Charge
Reading List Question 31 of 45 Submit Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge Zeff. Nuclear charge atomic number of the atom.
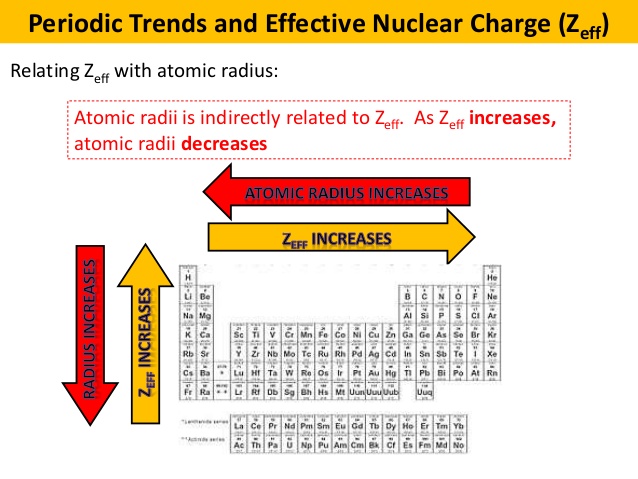
Atomic Radius Ionization Energy Question Socratic
The following probabilities are based on data collected from U.

. AnswerNuclear charge is the number of protons in the nucleus. The atomic numbers of your atoms are. Rank the following atoms in order of decreasing first ionization energies ie highest to lowest.
12 6 C C. Number of neutrons D. Helium has 2 protons in its nucleus so it has a nuclear charge of 0.
A CI BC CF D Ne E B. Which of the following atoms has the greatest nuclear charge. 11 all atoms in a given sample of an element contain the same number of.
N-14 C-12 H-2 He-4. Chemistry questions and answers. Which of the following atoms has the greatest nuclear charge.
14 7 N B. Al- 13 Ar- 18 Si- 14 Na- 11 Thus Ar has the greatest nuclear charge. Sodium has 11 electrons in its nucleus and therefore a nuclear charge of 19.
Whitch of the following atoms has the greatest nuclear charge. A Al B At C Si D Na. 4 2 He 4.
Because argon contains the most protons in its nucleus it has the highest nuclear charge. Up to 10 cash back Because chlorine is in the same period as phosphorus and sodium but has the most protons in its shell the most right within the same period it has the greatest effective nuclear charge. Which pair of elements form an ionic bond with each other.
Which is the atomic number of an atom with six valence electrons. Li Be Ba F. All atoms of one element have the same mass.
Which statement identifies a disadvantage to this location. What is the nuclear charge of an atom with a mass of 23 and atomic number of 11. The bonds in the compound MgSO 4 can be.
No charge 1 amu. Which of the following has the greatest nuclear charge. Which of the following atoms has the greatest nuclear charge.
All of the atoms of argon have the same A. Argon has 18 protons in its nucleus. A Al BAr CSi DNa.
Considering periodic trends valence electrons in which of the following atoms experience the greatest effective nuclear charge Zeff. Whitch of the following atoms has the greatest nuclear charge. Explain in terms of particles why statement A is no longer accepted.
So your answer is 2Ar Snapsolve. What can be determined if. Silicon has 14 electrons in its nucleus and therefore a nuclear charge of 28.
2 1 H D. Additionally because chlorine is in the same group as bromine but is higher up on the periodic table it has a greater effective nuclear charge making it. Which of the following atoms has the greatest nuclear charge.
Utah has the largest concentration of oil shale and tar sands in the United States. Number of nucleons 2. 2 October 2021 by lets tokmak.
A CI B С. Whitch of the following atoms has the greatest nuclear charge.

What Is Electricity What Is Electricity Electrons Protons

Atoms And Molecules Chemistry Classroom Chemistry Lessons Teaching Chemistry

Tetryonics 54 11 Modern Nuclear Isotope Theory Represents One Of The Greatest Foundational Errors Of Assumption In Mode Nuclear Energy Elements Quantum Leap
Comments
Post a Comment